Antinuclear Antibody Test Market Report
RA08330
Antinuclear Antibody Test Market, by Product (Reagents & Assay Kits, Systems and Software & Services), Technique (ELISA, Immunofluorescence Assay and Multiplex Assay), Application (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjogren’s syndrome, Scleroderma, and Others), End Use (Hospitals, Clinical Laboratories, Physician Office Laboratories, and Others), Regional Outlook (North America, Europe, Asia-Pacific, and LAMEA): Global Opportunity Analysis And Industry Forecast, 2022-2031
Global Antinuclear Antibody Test Market Analysis
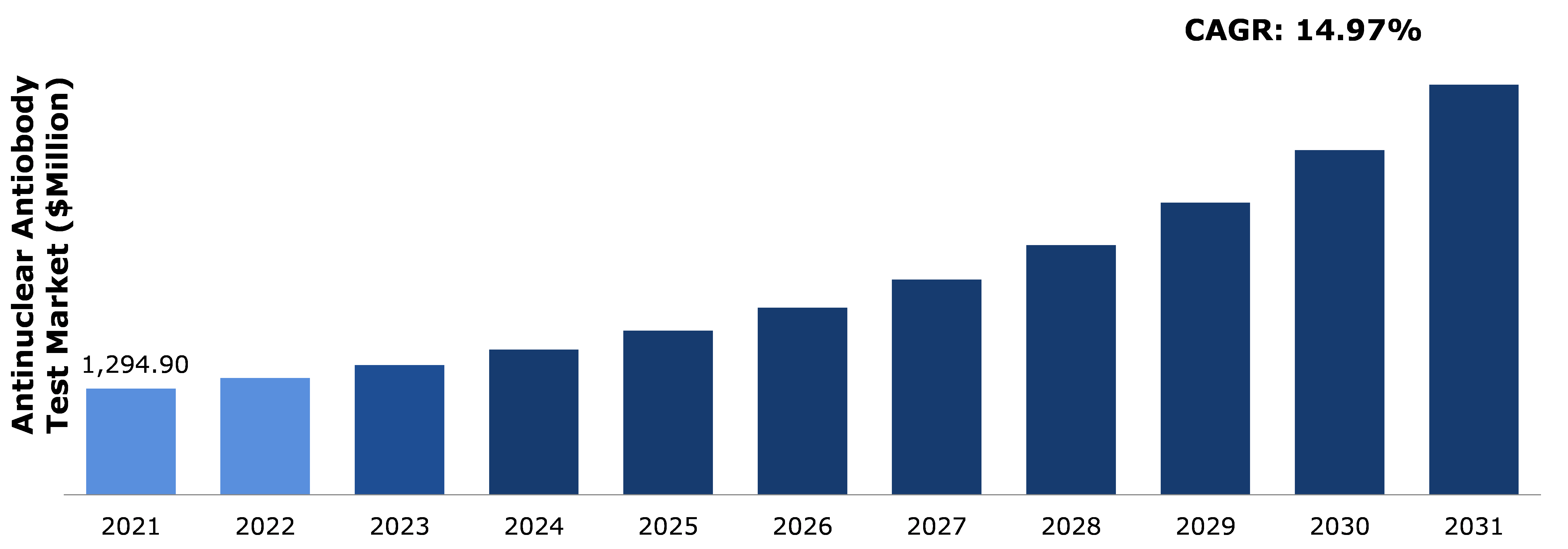
The global antinuclear antibody test market size was $1,294.90 million in 2021 and is predicted to grow with a CAGR of 14.97%, by generating a revenue of $4,999.40 million by 2031.
Global Antinuclear Antibody Test Market Synopsis
The antinuclear antibody (ANA) test is a blood test done to determine whether there are any autoimmune diseases that affect the body's tissues and organs. These exams look for antinuclear antibodies in the blood. Antibodies are proteins that the human immune system normally makes to fight foreign bodies such as viruses and bacteria. However, these antibodies start attacking their own healthy cells instead. It is fine to have a few antinuclear antibodies in the blood. However, the presence of a large number of antinuclear antibodies is a sign of having an autoimmune disease.
Indirect immunofluorescence, ELISA, and other procedures are some of the methods used to assess antinuclear antibodies. The cases of autoimmune diseases are increasing across the globe. The increasing incidence rate of autoimmune diseases is a serious threat to society. For instance, according to the National Stem Cell Foundation (NSCF), a non-profit organization that funds regenerative medicine and adult stem cell research, around 4% of the world population is affected by one of more than eighty different autoimmune diseases. The most common autoimmune diseases include type 1 diabetes, rheumatoid arthritis, multiple sclerosis, Crohn’s disease, lupus, psoriasis, and scleroderma. Antinuclear antibody tests are becoming more and more popular as a way to diagnose autoimmune diseases such as rheumatoid arthritis, Systemic Lupus Erythematosus, Sjogren's syndrome, and many more. The growing prevalence of autoimmune diseases is the main factor driving the demand for the antinuclear antibody test market.
A shortage of skilled lab technicians and diagnostic mistakes are the main factors anticipated to impede the growth of the global anti-nuclear antibody testing market. For instance, according to the American College of Rheumatology, anti-nuclear antibody test may yield false positive results in healthy individuals, who are free of any medical illnesses or those who are taking drugs like isoniazid and procainamide. According to the Herald Scholarly Open Access, the antinuclear antibody test is very sensitive for the diagnosis of an autoimmune disorder and may provide false results. It has been observed that 15% of healthy individuals have a positive antinuclear antibody test without autoimmune disorders.
The growing awareness and improving healthcare infrastructure in developing countries are anticipated to provide growth opportunities for the global antinuclear antibody test market during the forecast period. Furthermore, the rising number of diagnostic laboratories along with the increasing investment in lab automation is projected to provide growth opportunities for the global market.
According to regional analysis, the North America antinuclear antibody test market accounted for $461.00 million in 2021 and is predicted to grow with a CAGR of 13.85% in the projected timeframe. The growing incidence of autoimmune diseases across the region is driving the growth of the anti-nuclear antibody test market.
Antibody Antinuclear Overview
The ANA test is performed to check for the presence of antinuclear antibodies in the blood. The presence of antinuclear antibodies in the blood may indicate an individual might have an autoimmune condition. The immune system mistakenly attacks one's cells, tissues, and/or organs as a result of an autoimmune illness. Serious health issues may result from these conditions. Additionally, the immune system produces antibodies, which are proteins, to combat external invaders like viruses and bacteria. An antinuclear antibody, however, targets one's healthy cells.
COVID-19 Impact on Global Antinuclear Antibody Test Market
The COVID-19 pandemic is anticipated to have a positive effect on the market. Anti-nuclear antibody testing since COVID-19 patients have a high chance of developing autoimmune illnesses, which necessitates early diagnosis utilizing anti-nuclear antibody testing as a screening technique. For instance, according to a study conducted by the Department of Laboratory Medicine and Pathology at the University of Washington in the United States in 2020, 30% of COVID-19 patients had anti-nuclear antibodies that could be detected. Antinuclear antibody testing has also been the subject of wide research and clinical studies for the development of the COVID-19 vaccine and for the treatment of COVID-19, which will further fuel the market's expansion.
Increasing Cases of Autoimmune Diseases across the Globe is Fueling the Demand for ANA Test Market
The incidence of autoimmune diseases is rising across the world. However, according to medical practitioners, the cause of the onset of autoimmune disease is unknown. Individual own genes combined with environmental or infection might be the reason for the cause of the autoimmune disorder. In an autoimmune disorder, the body's immune system attacks one's healthy cells. The conditions include rheumatoid arthritis, type 1 diabetes, multiple sclerosis, Crohn’s disease, lupus, psoriasis, and scleroderma. Changing lifestyles and eating are linked to increased incidences of autoimmune diseases across the globe. Western food items such as fried chicken, and pizza, burgers are becoming popular worldwide. However, regular consumption of these food products could be one of the factors for the spike in cases of autoimmune diseases worldwide. For instance, according to Johns Hopkins University, autoimmune disease affects 3% of the U.S. population. The increasing incidence of autoimmune disease is the main factor driving the demand for the antinuclear antibody test market.
To know more about global methanol market drivers, get in touch with our analysts here.
Shortage of Skilled Lab technicians and High Cost of Advanced Lab Equipment May Hinder the Market Growth
Antinuclear antibody testing is a sensitive process and sometimes yields false results, even in healthy individuals. As a result, testing antinuclear antibodies require trained and skilled technicians. Furthermore, detecting and accurately predicting the result of the ANA test requires advanced lad equipment. The high cost of this equipment may hinder the market growth during the forecast period. Therefore, the shortage of skilled lab technicians and the high cost of advanced lab equipment may hinder the market growth.
Rising R&D Spending and Improving Healthcare Infrastructure is Predicated to Create More Growth Opportunities for the Market
Improving healthcare infrastructure and rising spending on laboratory automation are expected to provide growth opportunities for the global antinuclear antibody market. Furthermore, government initiatives and rising awareness about the autoimmune disease among people will further propel the demand for ANA tests during the forecast period. In addition, companies like Johnson & Johnson are increasingly spending on increasing product portfolios for autoimmune diseases. For instance, Johnson & Johnson acquired Momenta in October 2020 to increase its focus on autoimmune diseases. Johnson & Johnson now has rights to nipocalimab (M281), a clinically validated antibody. Nipocalimab could be used to treat autoimmune diseases. Growing R&D investment to develop novel treatments will provide growth opportunities for the ANA test market in the coming years.
To know more about global antinuclear antibody test market opportunities, get in touch with our analysts here.
Global Antinuclear Antibody Test Market Share, by Product
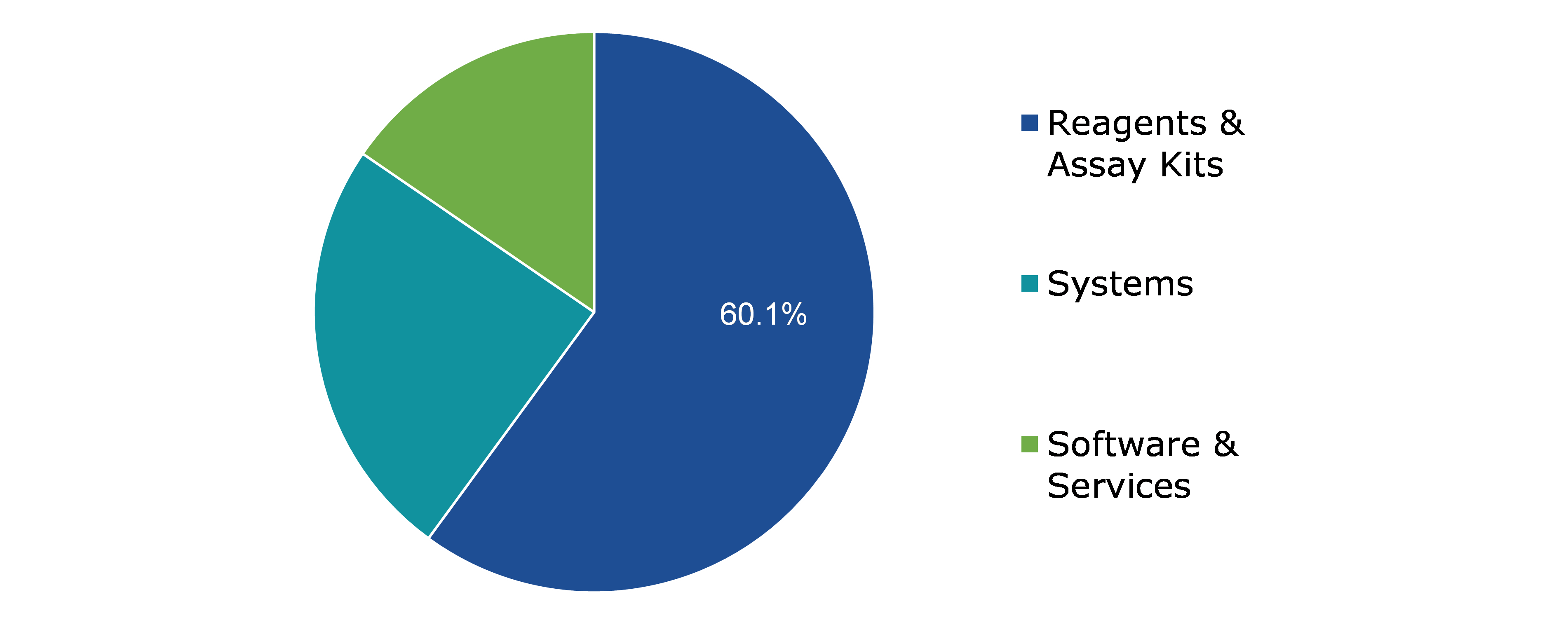
Based on product, the market has been divided into reagents & assay kits, systems, software & services. Among these, the reagents & assay kits sub-segment accounted for the highest market share in 2021 and estimated to show the fastest growth during the forecast period. System sub-segment is expected to significantly grow during the timeframe
Global Antinuclear Antibody Test Market Size, by Product, 2021
Source: Research Dive Analysis
The reagents & assay kits sub-type is anticipated to have a dominant market share and fastest growth generating a revenue of $3,153.10 million by 2031, growing from $777.70 million in 2021. The test kits are primarily used in life science research, drug discovery and development, environmental monitoring, and the study of disease pathways, as well as for various research and development purposes such as screening for potential drug candidates and evaluating biopharmaceutical production processes.
The ANA test detects the presence of antinuclear antibodies in the blood of an individual which adheres to reagent test cells, generating distinctive fluorescence patterns linked to specific autoimmune disorders The growing number of ANA tests owing to increasing awareness among people, improving healthcare infrastructure, and a broad array of applications is anticipated to enhance the market throughout the projected timeframe..
The systems sub-type is anticipated to show the second dominate market share and shall generate a revenue of $ 1,089.90 million by 2031, increasing from $ 317.30 million in 2021. Government bodies are focusing on improving healthcare infrastructure and increasing spending on diagnostic lab automation to minimize human error and false results. Furthermore, hospitals and existing diagnostic labs are upgrading testing equipment and devices to increase efficiency. Such factors are driving the demand systems in the market.
Global Antinuclear Antibody Test Market Trends, by Technique
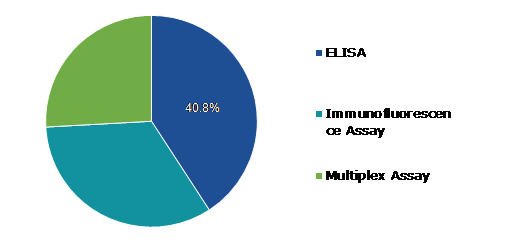
Based on technique, the market has been divided into ELISA, immunofluorescence assay, multiplex assay. Among these, the ELISA sub-segment accounted for highest revenue share in 2021 and is expected to be the fastest growing sub-segment.
Global Antinuclear Antibody Test Market Value, by Technique, 2021
Source: Research Dive Analysis
The ELISA sub-segment is anticipated to have a dominant market share and generate a revenue of $2,167.70 million by 2031, growing from $528.50 million in 2021. An ELISA or EIA test is also known as an enzyme-linked immunosorbent assay. The ANA ELISA is used to screen antinuclear antibodies in the specimen. Your body makes antibodies, which are proteins, in reaction to dangerous chemicals known as antigens. The ANA ELISA test is made to detect antibodies against histones, dsDNA, SS-B (La), SS-A (Ro), Smith, Smith/RNP, centromeric proteins, Scl-70, Jo-1, and other antigens extracted from the HEp-2 cell nucleus. Compared to ANA IFAANA, ELISA assays have lower sensitivities for systemic autoimmune rheumatic diseases (SARD). The increasing use of ELISA tests for screening antinuclear antibodies in the specimen is the main factor driving the market.
Global Antinuclear Antibody Test Market Growth, by Application
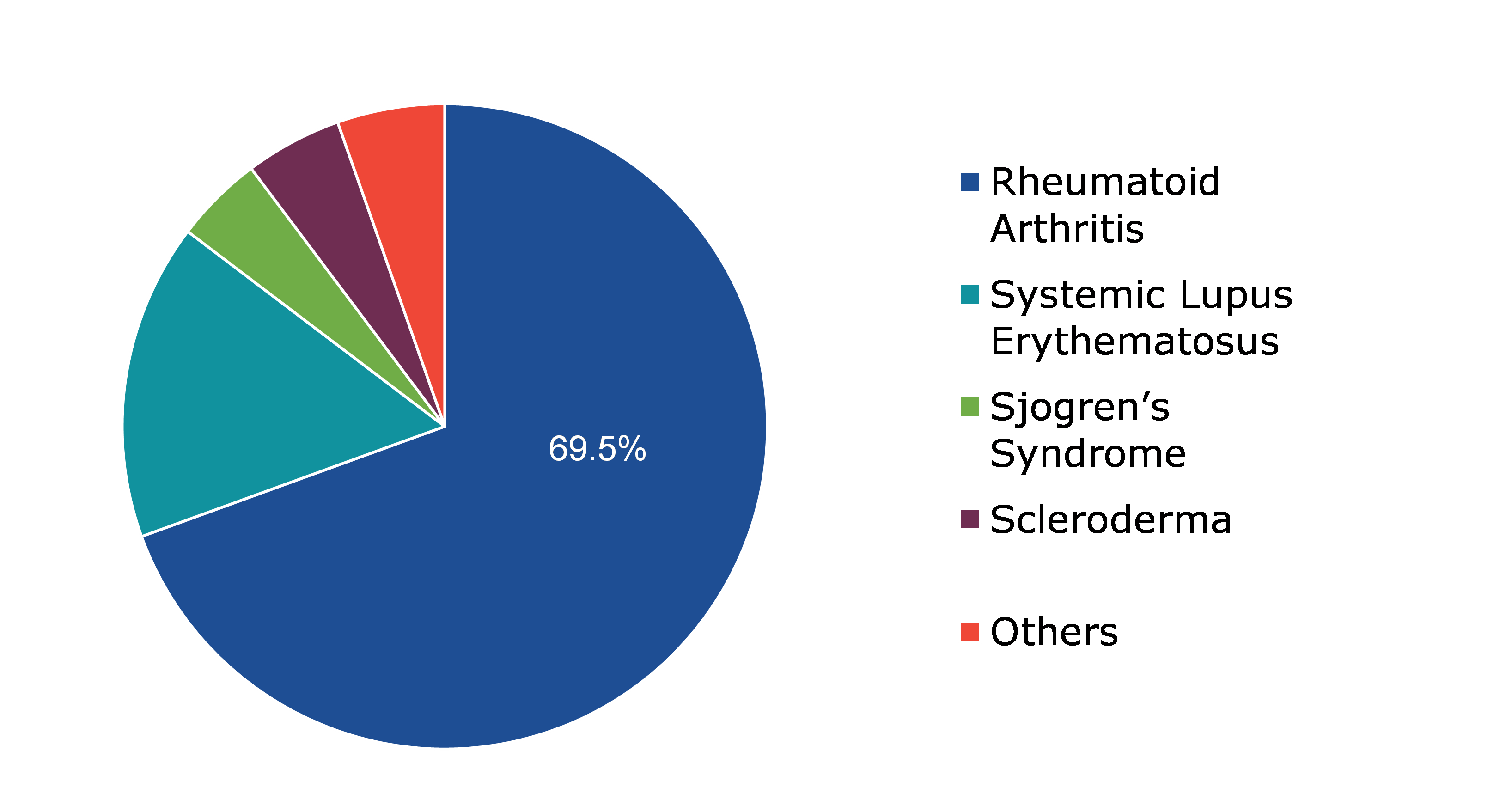
Based on application, the market has been divided into rheumatoid arthritis, systemic lupus erythematosus, sjogren’s syndrome, scleroderma and others. Among these, the rheumatoid arthritis sub-segment accounted for highest revenue share in 2021 and is expected to be the fastest growing sub-segment.
Global Antinuclear Antibody Test Market Share, by Application, 2021
Source: Research Dive Analysis
The rheumatoid arthritis sub-segment is anticipated to have a dominant market share and generate a revenue of $3,622.40 million by 2031, growing from $899.40 million in 2021. Rheumatoid arthritis, also known as RA, is an autoimmune and inflammatory condition in which your immune system unintentionally attacks healthy cells in the body, leading to inflammation (painful swelling) in the areas of the body affected. RA primarily targets the joints, typically several joints at once. Most cases of rheumatoid arthritis are in elderly people. The sub-segment market is anticipated to be driven by the growing geriatric population and increasing rheumatoid arthritis incidences.
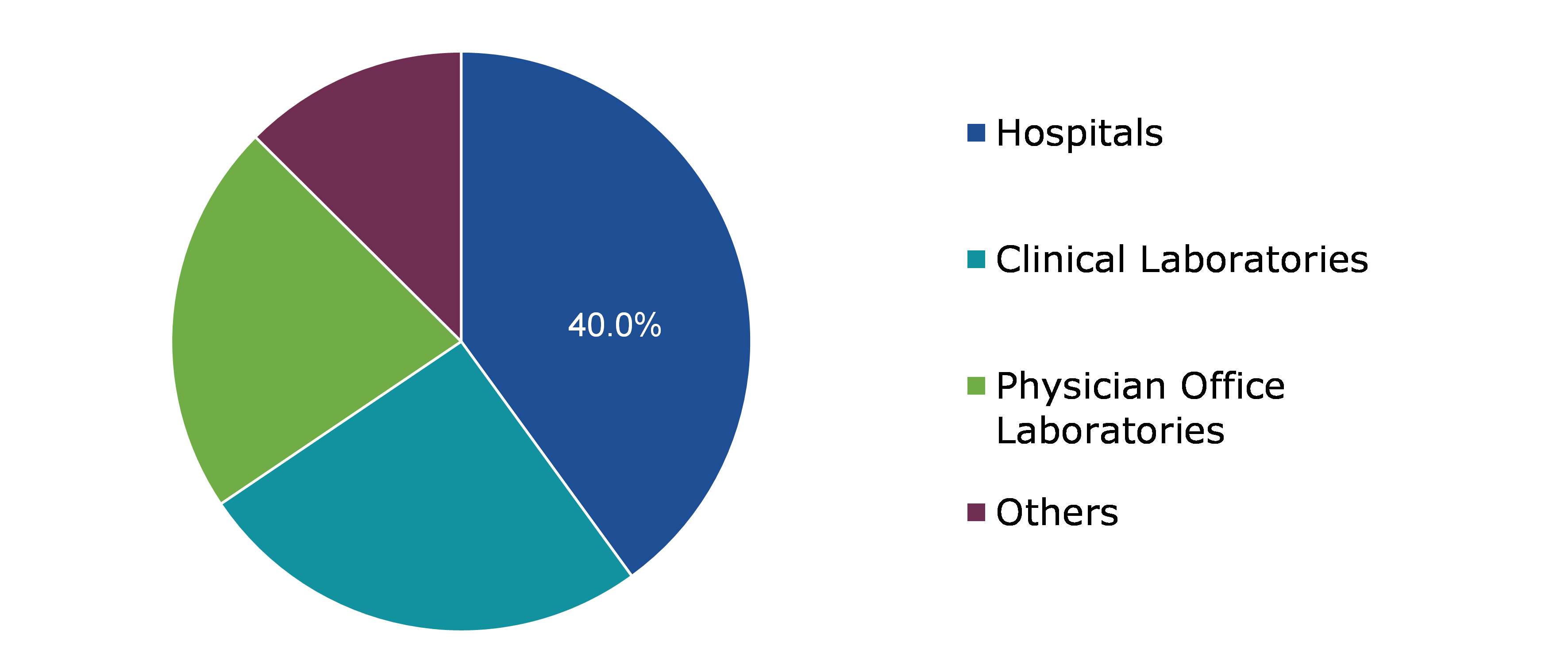
Global Antinuclear Antibody Test Market Size, by End use
Based on end use, the market has been divided into hospitals, clinical laboratories, physician office laboratories and others. Among these, the hospitals sub-segment accounted for highest revenue share in 2021.
Global Antinuclear Antibody Test Market Value, by End use, 2021
Source: Research Dive Analysis
The hospitals sub-segment is anticipated to have a dominant market share and generate a revenue of $1,907.60 million by 2031, growing from $517.90 million in 2021. High revenue share can be attributed to elements like rising autoimmune disease prevalence and the rising need for fast diagnosis. The hospital sub-segment market is anticipated to be driven by an increase in patient satisfaction, outpatient services, and enhanced home care facilities offered by hospitals throughout the forecasted period.
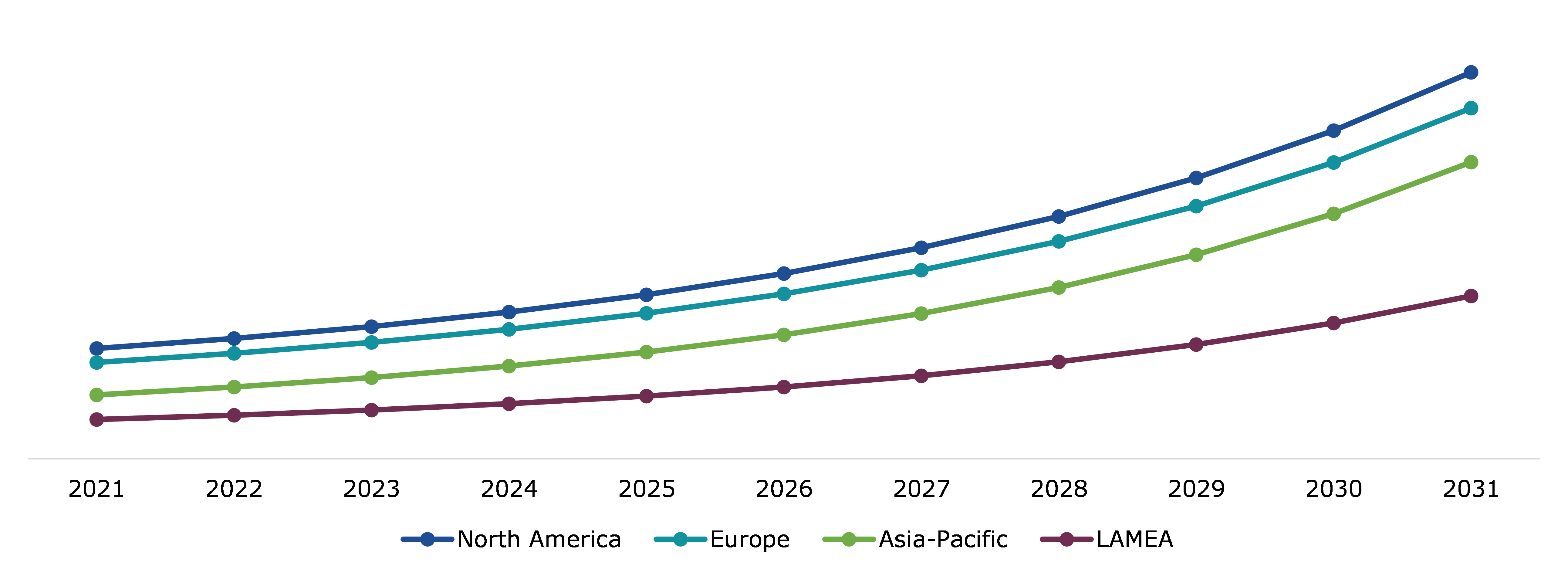
Global Antinuclear Antibody Test Market, Regional Insights
The antinuclear antibody test market was investigated across North America, Europe, Asia-Pacific, and LAMEA.
Global Antinuclear Antibody Test Market Size & Forecast, by Region, 2021-2031 (USD Million)
Source: Research Dive Analysis
The Market for Methanol in North America to be the Most Dominant
The North America antinuclear antibody test market accounted for $461.00 million in 2021 and is projected to grow with a CAGR of 13.85%. Throughout the entire projection period, North America is anticipated to lead the ANA market. The largest market share within the North American region is anticipated to be held by the United States over the study period. The region has a high demand for early diagnosis and efficient treatment due to several variables, including an aging population that is more susceptible to diseases due to weakened immunity.
The growing investment in the nation will accelerate progress in creating antinuclear antibody tests, further fueling market expansion during the study period. For instance, in November 2021, with the opening of the Colton Center for Autoimmunity in Pennsylvania, Stewart and Judy Colton donated US$10 million to the Perelman School of Medicine at the University of Pennsylvania to support ongoing autoimmune research and care. Increasing the number of ANA testing and rising R&D spending to develop novel tests will drive the market.
Competitive Scenario in the Global Antinuclear Antibody Test Market
Investment and agreement are common strategies followed by major market players. For instance, In June 2022, THERADIAG entered into an agreement with Quotient Limited, a commercial-stage diagnostics company, under which the two companies will partner to advance autoimmune diagnostics by leveraging Quotient’s MosaiQ platform.
Source: Research Dive Analysis
Some of the leading antinuclear antibody test market players are Erba Diagnostics, Bio-Rad Laboratories, Inc., Trinity Biotech Plc., Thermo Fisher Scientific, Antibodies Incorporated, EUROIMMUN Medizinische Labordiagnostika AG, Immuno Concepts NA Ltd., Inova Diagnostics, Inc., Alere Inc. and ZEUS Scientific, Inc.
| Aspect | Particulars |
| Historical Market Estimations | 2020 |
| Base Year for Market Estimation | 2021 |
| Forecast Timeline for Market Projection | 2022-2031 |
| Geographical Scope | North America, Europe, Asia-Pacific, LAMEA |
| Segmentation by Product |
|
| Segmentation by Technique |
|
| Segmentation by Application |
|
| Segmentation by End-use Industry |
|
| Key Companies Profiled |
|
Q1. What is the size of the global antinuclear antibody test market?
A. The size of the global antinuclear antibody test market was over $1,294.90 million in 2021 and is projected to reach $4,999.40 million by 2031.
Q2. Which are the major companies in the antinuclear antibody test market?
A. Alere Inc., Bio-Rad Laboratories, Inc., ERBA Diagnostics, Inc., Trinity Biotech plc, Thermo Fisher Scientific, Inc. are some of the key players in the global antinuclear antibody test market.
Q3. Which region, among others, possesses greater investment opportunities in the near future?
A. The North America region possesses great investment opportunities for investors to witness the most promising growth in the future.
Q4. What will be the growth rate of the Asia-Pacific antinuclear antibody test market?
A. Asia-Pacific antinuclear antibody test market is anticipated to grow at 17.1% CAGR during the forecast period.
Q5. What are the strategies opted by the leading players in this market?
A. Agreement and investment are the two key strategies opted by the operating companies in this market.
Q6. Which companies are investing more on R&D practices?
A. Erba Diagnostics is the company investing more on R&D activities for developing new products and technologies.
1.Research Methodology
1.1.Desk Research
1.2.Real time insights and validation
1.3.Forecast model
1.4.Assumptions and forecast parameters
1.5.Market size estimation
1.5.1.Top-down approach
1.5.2.Bottom-up approach
2.Report Scope
2.1.Market definition
2.2.Key objectives of the study
2.3.Report overview
2.4.Market segmentation
2.5.Overview of the impact of COVID-19 on Global antinuclear antibody test market
3.Executive Summary
4.Market Overview
4.1.Introduction
4.2.Growth impact forces
4.2.1.Drivers
4.2.2.Restraints
4.2.3.Opportunities
4.3.Market value chain analysis
4.3.1.List of raw material suppliers
4.3.2.List of manufacturers
4.3.3.List of distributors
4.4.Innovation & sustainability matrices
4.4.1.Technology matrix
4.4.2.Regulatory matrix
4.5.Porter’s five forces analysis
4.5.1.Bargaining power of suppliers
4.5.2.Bargaining power of consumers
4.5.3.Threat of substitutes
4.5.4.Threat of new entrants
4.5.5.Competitive rivalry intensity
4.6.PESTLE analysis
4.6.1.Political
4.6.2.Economical
4.6.3.Social
4.6.4.Technological
4.6.5.Environmental
4.7.Impact of COVID-19 on antinuclear antibody test market
4.7.1.Pre-COVID market scenario
4.7.2.Post-COVID market scenario
5.Antinuclear Antibody Test Market Analysis, by Product
5.1.Overview
5.2.Reagents & Assay Kits
5.2.1.Definition, key trends, growth factors, and opportunities
5.2.2.Market size analysis, by region, 2021-2031
5.2.3.Market share analysis, by country, 2021-2031
5.3.Systems
5.3.1.Definition, key trends, growth factors, and opportunities
5.3.2.Market size analysis, by region, 2021-2031
5.3.3.Market share analysis, by country, 2021-2031
5.4.Software & Services
5.4.1.Definition, key trends, growth factors, and opportunities
5.4.2.Market size analysis, by region, 2021-2031
5.4.3.Market share analysis, by country, 2021-2031
5.5.Research Dive Exclusive Insights
5.5.1.Market attractiveness
5.5.2.Competition heatmap
6.Antinuclear Antibody Test Market Analysis, by Technique
6.1.ELISA
6.1.1.Definition, key trends, growth factors, and opportunities
6.1.2.Market size analysis, by region, 2021-2031
6.1.3.Market share analysis, by country, 2021-2031
6.2.Immunofluorescence Assay
6.2.1.Definition, key trends, growth factors, and opportunities
6.2.2.Market size analysis, by region, 2021-2031
6.2.3.Market share analysis, by country, 2021-2031
6.3.Multiplex Assay
6.3.1.Definition, key trends, growth factors, and opportunities
6.3.2.Market size analysis, by region, 2021-2031
6.3.3.Market share analysis, by country, 2021-2031
6.4.Research Dive Exclusive Insights
6.4.1.Market attractiveness
6.4.2.Competition heatmap
7.Antinuclear Antibody Test Market Analysis, by Application
7.1.Rheumatoid Arthritis
7.1.1.Definition, key trends, growth factors, and opportunities
7.1.2.Market size analysis, by region, 2021-2031
7.1.3.Market share analysis, by country, 2021-2031
7.2.Systemic Lupus Erythematosus
7.2.1.Definition, key trends, growth factors, and opportunities
7.2.2.Market size analysis, by region, 2021-2031
7.2.3.Market share analysis, by country, 2021-2031
7.3.Sjogren’s Syndrome
7.3.1.Definition, key trends, growth factors, and opportunities
7.3.2.Market size analysis, by region, 2021-2031
7.3.3.Market share analysis, by country, 2021-2031
7.4.Scleroderma
7.4.1.Definition, key trends, growth factors, and opportunities
7.4.2.Market size analysis, by region, 2021-2031
7.4.3.Market share analysis, by country, 2021-2031
7.5.Others
7.5.1.Definition, key trends, growth factors, and opportunities
7.5.2.Market size analysis, by region, 2021-2031
7.5.3.Market share analysis, by country, 2021-2031
7.6.Research Dive Exclusive Insights
7.6.1.Market attractiveness
7.6.2.Competition heatmap
8.Antinuclear Antibody Test Market Analysis, by End-use
8.1.Hospitals
8.1.1.Definition, key trends, growth factors, and opportunities
8.1.2.Market size analysis, by region, 2021-2031
8.1.3.Market share analysis, by country, 2021-2031
8.2. Clinical Laboratories
8.2.1.Definition, key trends, growth factors, and opportunities
8.2.2.Market size analysis, by region, 2021-2031
8.2.3.Market share analysis, by country, 2021-2031
8.3.Physician Office Laboratories
8.3.1.Definition, key trends, growth factors, and opportunities
8.3.2.Market size analysis, by region, 2021-2031
8.3.3.Market share analysis, by country, 2021-2031
8.4.Others
8.4.1.Definition, key trends, growth factors, and opportunities
8.4.2.Market size analysis, by region, 2021-2031
8.4.3.Market share analysis, by country, 2021-2031
8.5.Research Dive Exclusive Insights
8.5.1.Market attractiveness
8.5.2.Competition heatmap
9.Antinuclear Antibody Test Market, by Region
9.1.North America
9.1.1.U.S.
9.1.1.1.Market size analysis, by Product, 2021-2031
9.1.1.2.Market size analysis, by Technique, 2021-2031
9.1.1.3.Market size analysis, by Application, 2021-2031
9.1.1.4.Market size analysis, by End-use, 2021-2031
9.1.2.Canada
9.1.2.1.Market size analysis, by Product, 2021-2031
9.1.2.2.Market size analysis, by Technique, 2021-2031
9.1.2.3.Market size analysis, by Application, 2021-2031
9.1.2.4.Market size analysis, by End-use, 2021-2031
9.1.3.Mexico
9.1.3.1.Market size analysis, by Product, 2021-2031
9.1.3.2.Market size analysis, by Technique, 2021-2031
9.1.3.3.Market size analysis, by Application, 2021-2031
9.1.3.4.Market size analysis, by End-use, 2021-2031
9.1.4.Research Dive Exclusive Insights
9.1.4.1.Market attractiveness
9.1.4.2.Competition heatmap
9.2.Europe
9.2.1.Germany
9.2.1.1.Market size analysis, by Product, 2021-2031
9.2.1.2.Market size analysis, by Technique, 2021-2031
9.2.1.3.Market size analysis, by Application, 2021-2031
9.2.1.4.Market size analysis, by End-use, 2021-2031
9.2.2.UK
9.2.2.1.Market size analysis, by Product, 2021-2031
9.2.2.2.Market size analysis, by Technique, 2021-2031
9.2.2.3.Market size analysis, by Application, 2021-2031
9.2.2.4.Market size analysis, by End-use, 2021-2031
9.2.3.France
9.2.3.1.Market size analysis, by Product, 2021-2031
9.2.3.2.Market size analysis, by Technique, 2021-2031
9.2.3.3.Market size analysis, by Application, 2021-2031
9.2.3.4.Market size analysis, by End-use, 2021-2031
9.2.4.Spain
9.2.4.1.Market size analysis, by Product, 2021-2031
9.2.4.2.Market size analysis, by Technique, 2021-2031
9.2.4.3.Market size analysis, by Application, 2021-2031
9.2.4.4.Market size analysis, by End-use, 2021-2031
9.2.5.Italy
9.2.5.1.Market size analysis, by Product, 2021-2031
9.2.5.2.Market size analysis, by Technique, 2021-2031
9.2.5.3.Market size analysis, by Application, 2021-2031
9.2.5.4.Market size analysis, by End-use, 2021-2031
9.2.6.Rest of Europe
9.2.6.1.Market size analysis, by Product, 2021-2031
9.2.6.2.Market size analysis, by Technique, 2021-2031
9.2.6.3.Market size analysis, by Application, 2021-2031
9.2.6.4.Market size analysis, by End-use, 2021-2031
9.2.7.Research Dive Exclusive Insights
9.2.7.1.Market attractiveness
9.2.7.2.Competition heatmap
9.3.Asia-Pacific
9.3.1.China
9.3.1.1.Market size analysis, by Product, 2021-2031
9.3.1.2.Market size analysis, by Technique, 2021-2031
9.3.1.3.Market size analysis, by Application, 2021-2031
9.3.1.4.Market size analysis, by End-use, 2021-2031
9.3.2.Japan
9.3.2.1.Market size analysis, by Product, 2021-2031
9.3.2.2.Market size analysis, by Technique, 2021-2031
9.3.2.3.Market size analysis, by Application, 2021-2031
9.3.2.4.Market size analysis, by End-use, 2021-2031
9.3.3.India
9.3.3.1.Market size analysis, by Product, 2021-2031
9.3.3.2.Market size analysis, by Technique, 2021-2031
9.3.3.3.Market size analysis, by Application, 2021-2031
9.3.3.4.Market size analysis, by End-use, 2021-2031
9.3.4.Australia
9.3.4.1.Market size analysis, by Product, 2021-2031
9.3.4.2.Market size analysis, by Technique, 2021-2031
9.3.4.3.Market size analysis, by Application, 2021-2031
9.3.4.4.Market size analysis, by End-use, 2021-2031
9.3.5.South Korea
9.3.5.1.Market size analysis, by Product, 2021-2031
9.3.5.2.Market size analysis, by Technique, 2021-2031
9.3.5.3.Market size analysis, by Application, 2021-2031
9.3.5.4.Market size analysis, by End-use, 2021-2031
9.3.6.Rest of Asia-Pacific
9.3.6.1.Market size analysis, by Product, 2021-2031
9.3.6.2.Market size analysis, by Technique, 2021-2031
9.3.6.3.Market size analysis, by Application, 2021-2031
9.3.6.4.Market size analysis, by End-use, 2021-2031
9.3.7.Research Dive Exclusive Insights
9.3.7.1.Market attractiveness
9.3.7.2.Competition heatmap
9.4.LAMEA
9.4.1.Brazil
9.4.1.1.Market size analysis, by Product, 2021-2031
9.4.1.2.Market size analysis, by Technique, 2021-2031
9.4.1.3.Market size analysis, by Application, 2021-2031
9.4.1.4.Market size analysis, by End-use, 2021-2031
9.4.2.Saudi Arabia
9.4.2.1.Market size analysis, by Product, 2021-2031
9.4.2.2.Market size analysis, by Technique, 2021-2031
9.4.2.3.Market size analysis, by Application, 2021-2031
9.4.2.4.Market size analysis, by End-use, 2021-2031
9.4.3.UAE
9.4.3.1.Market size analysis, by Product, 2021-2031
9.4.3.2.Market size analysis, by Technique, 2021-2031
9.4.3.3.Market size analysis, by Application, 2021-2031
9.4.3.4.Market size analysis, by End-use, 2021-2031
9.4.4.South Africa
9.4.4.1.Market size analysis, by Product, 2021-2031
9.4.4.2.Market size analysis, by Technique, 2021-2031
9.4.4.3.Market size analysis, by Application, 2021-2031
9.4.4.4.Market size analysis, by End-use, 2021-2031
9.4.5.Rest of LAMEA
9.4.5.1.Market size analysis, by Product, 2021-2031
9.4.5.2.Market size analysis, by Technique, 2021-2031
9.4.5.3.Market size analysis, by Application, 2021-2031
9.4.5.4.Market size analysis, by End-use, 2021-2031
9.4.6.Research Dive Exclusive Insights
9.4.6.1.Market attractiveness
9.4.6.2.Competition heatmap
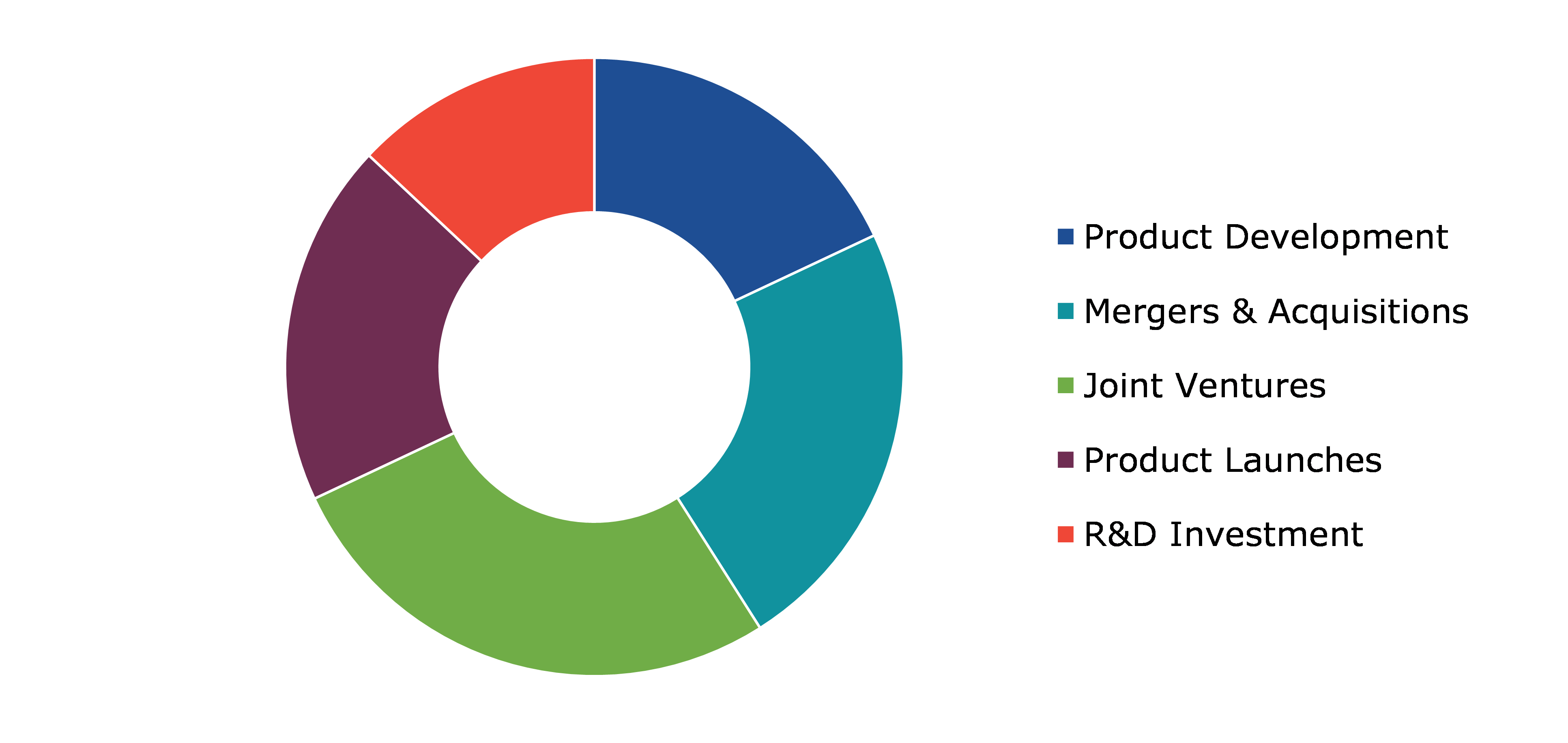
10.Competitive Landscape
10.1.Top winning strategies, 2021
10.1.1.By strategy
10.1.2.By year
10.2.Strategic overview
10.3.Market share analysis, 2021
11.Company Profiles
11.1.Erba Diagnostics
11.1.1.Overview
11.1.2.Business segments
11.1.3.Product portfolio
11.1.4.Financial performance
11.1.5.Recent developments
11.1.6.SWOT analysis
11.2.Bio-Rad Laboratories, Inc.
11.2.1.Overview
11.2.2.Business segments
11.2.3.Product portfolio
11.2.4.Financial performance
11.2.5.Recent developments
11.2.6.SWOT analysis
11.3.Trinity Biotech Plc.
11.3.1.Overview
11.3.2.Business segments
11.3.3.Product portfolio
11.3.4.Financial performance
11.3.5.Recent developments
11.3.6.SWOT analysis
11.4.Thermo Fisher Scientific
11.4.1.Overview
11.4.2.Business segments
11.4.3.Product portfolio
11.4.4.Financial performance
11.4.5.Recent developments
11.4.6.SWOT analysis
11.5.Antibodies Incorporated
11.5.1.Overview
11.5.2.Business segments
11.5.3.Product portfolio
11.5.4.Financial performance
11.5.5.Recent developments
11.5.6.SWOT analysis
11.6.EUROIMMUN Medizinische Labordiagnostika AG
11.6.1.Overview
11.6.2.Business segments
11.6.3.Product portfolio
11.6.4.Financial performance
11.6.5.Recent developments
11.6.6.SWOT analysis
11.7.Immuno Concepts NA Ltd.
11.7.1.Overview
11.7.2.Business segments
11.7.3.Product portfolio
11.7.4.Financial performance
11.7.5.Recent developments
11.7.6.SWOT analysis
11.8.Inova Diagnostics, Inc.
11.8.1.Overview
11.8.2.Business segments
11.8.3.Product portfolio
11.8.4.Financial performance
11.8.5.Recent developments
11.8.6.SWOT analysis
11.9.ZEUS Scientific, Inc.
11.9.1.Overview
11.9.2.Business segments
11.9.3.Product portfolio
11.9.4.Financial performance
11.9.5.Recent developments
11.9.6.SWOT analysis
11.10.Alere Inc.
11.10.1.Overview
11.10.2.Business segments
11.10.3.Product portfolio
11.10.4.Financial performance
11.10.5.Recent developments
11.10.6.SWOT analysis
12.Appendix
12.1.Parent & peer market analysis
12.2.Premium insights from industry experts
12.3.Related reports
Generally, antinuclear antibodies attack the tissues in a human body— specifically targeting the nucleus of each cell. An antinuclear antibody test is used to examine whether a person has an autoimmune disorder, a condition in which the immune system attacks healthy cells in the body. Nowadays, the prevalence of autoimmune diseases is increasing rapidly. However, studies based on systematic data are inadequate, so it is still unclear whether the rise in cases of autoimmune diseases is merely owing to developments in diagnosis and reporting.
There are a number of factors that can trigger an autoimmune disease, such as poor diet, stress, lack of workout, inadequate sleep, and smoking. As all these factors are highly prevalent in today’s generation, the rate of occurrence of autoimmune diseases has also surged. This has greatly accelerated the growth of the antinuclear antibody test market in recent years.
Newest Insights in the Antinuclear Antibody Test Market
As per a report by Research Dive, the global antinuclear antibody test market is expected to grow with a 14.97% CAGR and surpass $4,999.40 million in the 2022–2031 timeframe. The North America antinuclear antibody test market is expected to perceive significant growth with a CAGR of 13.85% in the years to come. This is because of the growing incidence of autoimmune diseases in the region. Moreover, there is high demand for early diagnosis and effective treatment of autoimmune diseases in this region owing to numerous factors such as the growing elderly population which is more vulnerable to diseases due to weak immunity. In addition, rising investments in the U.S. for developing advanced antinuclear antibody tests is predicted to drive the growth of the regional market.
How are the Market Players Responding to the Rising Demand for Antinuclear Antibody Tests?
A significant increase in the cases of chronic ailments across the globe is propelling the demand for antinuclear antibody tests. Market players are greatly investing in innovative research and developments to cater to the rising demand for antinuclear antibody tests. Some of the foremost players in the antinuclear antibody test market are Bio-Rad Laboratories, Inc., Trinity Biotech Plc., Immuno Concepts NA Ltd., Thermo Fisher Scientific, EUROIMMUN Medizinische Labordiagnostika AG, Inova Diagnostics, Inc., Erba Diagnostics, Alere Inc., ZEUS Scientific, Inc, Antibodies Incorporated, and others. These players are focused on planning and devising tactics such as mergers and acquisitions, collaborations, novel advances, and partnerships to reach a notable position in the global market.
For instance,
- In August 2018, Inova Diagnostics, a global frontrunner in autoimmune disease diagnostic systems and reagents for the clinical laboratory, announced the clearance of QUANTA Flash® HMGCR by the U.S. Food and Drug Administration (FDA).
- In October 2020, Thermo Fisher Scientific, a key player in the antinuclear antibody test industry, developed two novel SARS-CoV-2 antibody tests termed: the Thermo Scientific OmniPATH COVID-19 Total Antibody ELISA test and the Thermo Scientific EliA SARS-CoV-2-Sp1 IgG test.
- In June 2022, Thermo Fisher Scientific, Inc., an American supplier of scientific instrumentation, reagents and consumables, and software services, obtained FDA approval for Autoimmune Disease Test - EliA RNA Polymerase III and EliA Rib-P blood tests - developed to detect Systemic Sclerosis (SSc) and Systemic Lupus Erythematosus (SLE).
COVID-19 Impact on the Global Antinuclear Antibody Test Market
The outbreak of the coronavirus pandemic in 2020 has optimistically impacted the global antinuclear antibody test market. During the pandemic period, an unpredicted surge has been observed in the demand for antinuclear antibody tests in healthcare centers as COVID-19 patients have greater chances of acquiring autoimmune diseases. The antinuclear antibody test market observed massive growth during the disastrous situations of the pandemic due to the rising need for early diagnosis tests, which boosted the use of antinuclear antibody tests. As per market experts, the global market is projected to observe massive growth, even after the relaxation of the pandemic, in the forthcoming years.
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com