Left Atrial Appendage (LAA) Closure Device Market Report
RA08587
Left Atrial Appendage (LAA) Closure Device Market by Product (Epicardial LAA Devices and Endocardial LAA Closure Devices) End-use (Hospitals, Ambulatory Surgery Centers and Others), and Regional Analysis (Europe, North America, Asia-Pacific, and LAMEA): Global Opportunity Analysis and Industry Forecast, 2022-2031
Global Left Atrial Appendage (LAA) Closure Device Market analysis
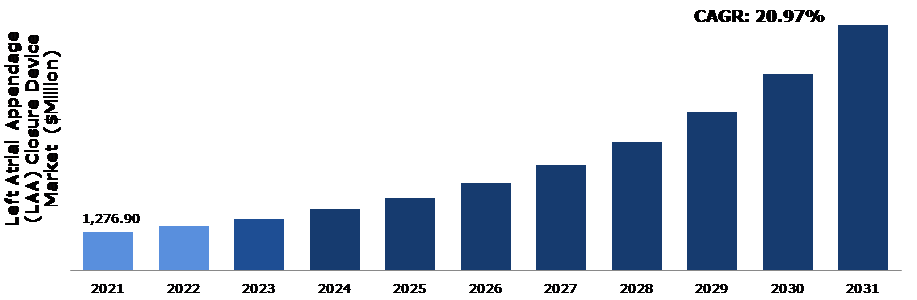
The global left atrial appendage (LAA) closure device market size was $1,276.90 million in 2021 and is predicted to grow with a CAGR of 20.97%, by generating a revenue of $8,218.10 million by 2031.
Left Atrial Appendage (LAA) Closure Device Market Synopsis
It has been discovered that the majority of strokes connected to atrial fibrillation are caused by thrombus development mostly from the left atrial appendage (LAA). Atrial fibrillation (AF), one of the most prevalent arrhythmias that affects about 2% of people globally. Atrial fibrillation (AF), a disorder that is on the rise throughout the world, is known to have a major negative influence on health, as well as long-term impacts disability and mortality. According to research, surgical removal and exclusion of the left atrial appendage are just as safe and effective as treating atrial fibrillation medically to prevent strokes. Atrial fibrillation's rising prevalence is boosting the market for appendage management. The need for appendage treatment is due to the rising prevalence of atrial fibrillation, which is one of the most difficult conditions considered under cardiology. The growing prevalence of atrial fibrillation is result of a high-calorie diet, smoking, and inactivity. The use of medications to treat atrial fibrillation is shown to be decreasing. In response to the negative effects of the medications, this alteration of left atrial appendage (LAA) closure device has been made. These markets are extremely attractive for medical device businesses due to changes in lifestyle, an increase in the prevalence of atrial fibrillation, better access to healthcare services, and a growth in demand for high-quality healthcare.
However, some factors such as high cost associated with the left atrial appendage (LAA) closure device can restrain the market growth. The time and cost invested during research and product development is resulting into the high cost of the left atrial appendage closure device.
Due to the healthcare industry’s rapid expansion and increased need for advanced technological solutions for treatment of atrial fibrillation related complications, the demand has increased significantly across globe for the Left Atrial Appendage (LAA) Closure Devices. Hospitals and other healthcare organizations are adopting new advancements that are available in the market to reduce the casualties of patients who have atrial fibrillation and are connected with high stroke risk. The global Left Atrial Appendage (LAA) Closure Device market demand is projected to see growth potential as a result of these factors.
Left Atrial Appendage (LAA) Closure Device Overview
Devices to close the left atrial appendage were developed as a substitute treatment for patients with atrial fibrillation to reduce their risk of stroke. The left atrial appendage is closed off with left atrial appendage closure devices, which prevents blood clots from forming and eliminates the need for the patient to take blood-thinning medication. As the prevalence of atrial fibrillation is expected to rise throughout the forecast period, competitors in the market are sponsoring trials and research to demonstrate the safety and efficacy of their respective devices.
COVID-19 Impact on Left Atrial Appendage (LAA) Closure Device Market
The rise in COVID-19 impact on left atrial appendage (LAA) closure device market cases is expected to drive the market, which can wean patients off of anticoagulation and may eliminate the need for frequent coumadin clinic visits for international normalized ratio (INR) testing, which is necessary to keep the medication at therapeutic levels. The two main drivers anticipated to drive the growth of the worldwide left atrial appendage closure devices market are the increasing number of people with atrial fibrillation (AF) and the ageing population. The major factor anticipated to drive the market growth during the study period is the efforts being made by governments across the globe to raise public awareness for the necessity of left atrial appendage closure devices during a pandemic.
Additionally, the COVID-19 pandemic is not expected to have any impact on the market for LAA closure devices, which is expected to drive the market significantly. The main drivers projected to boost the market expansion are the government's support for medical device approvals, the rise in the number of ambulatory clinical facilities throughout the world, and breakthroughs by well-known industry participants. For instance, in August 2021, Abbott Laboratories a multinational medical devices and health care company received the US FDA approval for Amplatzer Left Atrial Appendage Occular. The purpose of these devices is to treat atrial fibrillation. This approval will help the company increase product sales and take a large market share in the United States.
Rising Prevalence of Atrial Fibrillation is Expected to Drive the Global Left Atrial Appendage (LAA) Closure Device Market Growth
The increasing prevalence of atrial fibrillation is expected to increase the demand for left atrial appendage closure devices. For instance, the National Library of Medicine published a study titled Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge in January 2020. The most common cardiac arrhythmia is atrial fibrillation. This condition is expected to affect 6-12 million people in the United States by 2050, and 17.9 million people in the middle east by 2060. Atrial fibrillation is the potential cause of ischemic stroke, causing a significant impact on morbidity and mortality. Furthermore, the aging population is increasing the disease burden of atrial fibrillation because elderly individuals are more likely to be exposed to arrhythmia, ischemic stroke disease, and atrial fibrillation.
High Cost of Left Atrial Appendage (LAA) Closure Device may Restrain the Growth of the Global Left Atrial Appendage (LAA) Closure Device Market
High Cost of left atrial appendage (LAA) closure device is expected to restrain market expansion in the upcoming years. The overall cost of the systems is increasing as a result of ongoing technological advancement and the incorporation of contemporary technology. Customers are choosing alternate methods as a result, which is restraining the global market for left atrial appendage (LAA) closure market growth.
The Expanding Healthcare Sector in Developing Countries may Open New Opportunities in the Global Left Atrial Appendage (LAA) Closure Device Market
The healthcare industry in the nation is one of the most significant areas that contributes to the development of the country, and developing nations like India, Brazil, Myanmar, Thailand, Turkey, and others are concentrating more on it. Hospitals and other healthcare organizations are adopting new advancements that are available in the market to reduce the casualties of patients who are suffering from atrial fibrillation and are connected with a stroke risk. The global left atrial appendage (LAA) closure device market opportunity is projected to see growth potential as a result of these factors.
Global Left Atrial Appendage (LAA) Closure Device Market Share, by Product
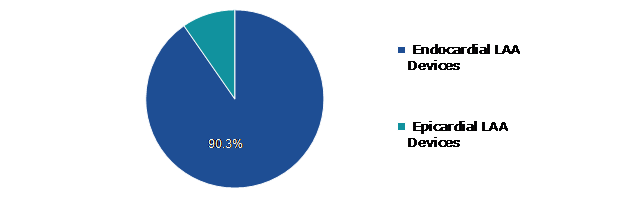
Based on Product, left atrial appendage (LAA) closure device market is further divided into Epicardial LAA devices and Endocardial LAA devices. Among these, Endocardial LAA devices sub-segment is expected to have dominating market share during the forecast period.
Global Left Atrial Appendage (LAA) Closure Device Market Size, by Product, 2021
The endocardial LAA devices sub-segment is anticipated to have a dominant market share and generate a revenue of $7,588.00 million by 2031, growing from $1,153.50 million in 2021. Comparing Endocardial LAA closure devices to Epicardial LAA closure devices, Endocardial LAA closure devices had lower rates of complications, shorter hospital stays, and fewer readmissions. According to the clinical investigations the Endocardial LAA devices offer improved safety and effectiveness in the management of stroke and the reduction of bleeding. This factor is expected to drive the growth of the left atrial appendage (LAA) closure device market growth in the analysis period.
The epicardial LAA devices sub-type is anticipated to show the second fastest growth and shall generate a revenue of $630.10 million by 2031, increasing from $123.40 million in 2021. Epicardial LAA devices have recently emerged as a successful solution for stroke control in patients with atrial fibrillation who are non-compliant with long-term oral anticoagulation medication. Clinical results show that epicardial LAA devices help to reduce the burden of atrial fibrillation and are associated with an early and constant drop in systemic blood pressure on short-term follow-up. Furthermore, epicardial LAA devices reduce the requirement for antihypertensive drugs more than endocardial LAA devices.
Global Left Atrial Appendage (LAA) Closure Device Market Value, by End-use
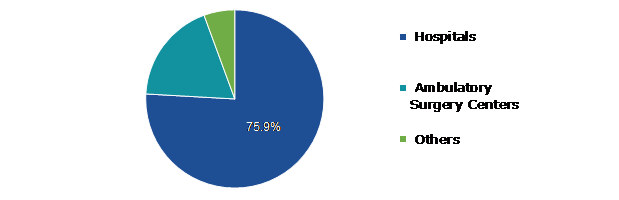
Based on end-use, the global Left Atrial Appendage (LAA) Closure Device market is further divided into hospitals, ambulatory surgery centers and others. Among these, hospitals sub-segment is expected to account for highest revenue share.
Global Left Atrial Appendage (LAA) Closure Device Market Growth, by End-Use 2021
Hospitals sub-segment is anticipated to have a dominant market share and generate a revenue of $6,499.40 million by 2031, growing from $969.00 million in 2021. This is due to the increased preference for LAA surgeries and adoption of left atrial appendage closure devices at hospitals. The preference for hospitals is predicted to increase due to the availability of cutting-edge medical technologies, certified medical personnel, and growing use of the most recent left atrial appendage closure devices. Additionally, hospitals' increased predilection for LAA surgeries and adoption of left atrial appendage closure devices. The availability of modern medical technology, skilled medical staff, and the expanding usage of the newest left atrial appendage closure devices are expected to boost hospital segment.
Global Left Atrial Appendage (LAA) Closure Device Market Share, Regional Insights
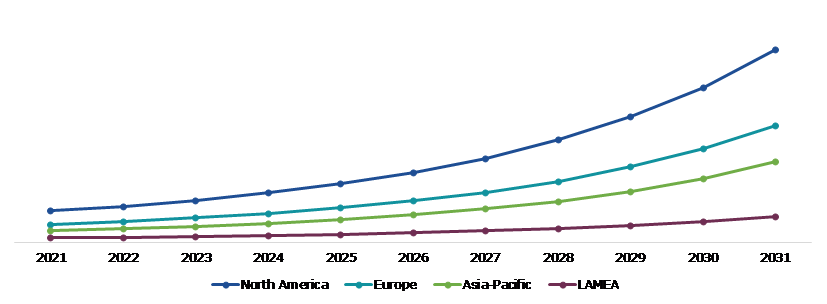
Global Left Atrial Appendage (LAA) Closure Device market was investigated across Europe, North America, Asia-Pacific, and LAMEA.
Global Left Atrial Appendage (LAA) Closure Device Market Size and Forecast, by Region, 2021-2031 (USD Million)
The Left Atrial Appendage (LAA) Closure Device Market in North America Region is Expected to be Dominant
The North America left atrial appendage (LAA) closure device market analysis accounted $615.50 million in 2021 and is projected to grow with a CAGR of 20.51%. The market for left atrial appendage (LAA) closure device market trend in North America has experienced significant expansion, mostly due to important factors such the need of advanced solutions for the treatment of atrial fibrillation and related complications. Some of the factors that are anticipated to drive the growth of the left atrial appendage (LAA) closure device market growth in the North American region include an ageing population and an increase in the number of patients in these areas.
Global Left Atrial Appendage (LAA) Closure Device Market Competitive Scenario
Some product advancements, and technological advancements are the common strategies followed by major market players. For instance, in April 2021, AtriCure, Inc. A leading specialist in the management of left atrial extensions and atrial fibrillation therapy stated that the US FDA had approved the EPi-Sense system for the treatment of patients with chronic persistent atrial fibrillation. The market for left atrial appendage (LAA) closure devices may experience increased sales as a result of this new development.
Leading companies in Left Atrial Appendage (LAA) Closure Device market are Boston Scientific Corporation, Abbott, Lifetech Scientific, ATRICURE, INC., SentreHEART, Inc., Medical Device Business Services, Inc., Occlutech, Cardia, Inc., Johnson & Johnson Private Limited, and Lepu Medical Technology Co., Ltd.
| Aspect | Particulars |
| Historical Market Estimations | 2020 |
| Base Year for Market Estimation | 2021 |
| Forecast timeline for Market Projection | 2022-2031 |
| Geographical Scope | Europe, North America, Asia-Pacific, LAMEA |
| Segmentation by Product
|
|
| Segmentation by End-use |
|
| Key Companies Profiled |
|
Q1. What is the size of the Left Atrial Appendage (LAA) Closure Device market?
A. The size of the global left atrial appendage (LAA) closure device market size was over $ 1,276.90 million in 2021 and is projected to reach $8,218.10 million by 2031
Q2. Which are the leading companies in the Left Atrial Appendage (LAA) Closure Device market?
A. Boston Scientific Corporation, Abbott, Lifetech Scientific are some of the key players in the global Left Atrial Appendage (LAA) Closure Device market.
Q3. Which region possesses greater investment opportunities in the coming future?
A. Asia- Pacific possesses great investment opportunities for the investors to witness the most promising growth in the coming years.
Q4. What is the growth rate of the Asia-Pacific market?
A. The Asia-Pacific left atrial appendage (LAA) closure device market share is anticipated to grow at 22.23% CAGR during the forecast period.
Q5. What are the strategies opted by the leading players in this market?
A. Product advancement and technological advancement are the key strategies opted by the operating companies in this market.
Q6. Which companies are investing more on R&D practices?
A. AtriCure, Inc. and Abbott are investing more on R&D activities for developing new products and technologies.
1.Research Methodology
1.1.Desk Research
1.2.Real time insights and validation
1.3.Forecast model
1.4.Assumptions and forecast parameters
1.5.Market size estimation
1.5.1.Top-down approach
1.5.2.Bottom-up approach
2.Report Scope
2.1.Market definition
2.2.Key objectives of the study
2.3.Report overview
2.4.Market segmentation
2.5.Overview of the impact of COVID-19 on Global left atrial appendage (LAA) closure device market
3.Executive Summary
4.Market Overview
4.1.Introduction
4.2.Growth impact forces
4.2.1.Drivers
4.2.2.Restraints
4.2.3.Opportunities
4.3.Market value chain analysis
4.3.1.List of raw material suppliers
4.3.2.List of manufacturers
4.3.3.List of distributors
4.4.Innovation & sustainability matrices
4.4.1.Technology matrix
4.4.2.Regulatory matrix
4.5.Porter’s five forces analysis
4.5.1.Bargaining power of suppliers
4.5.2.Bargaining power of consumers
4.5.3.Threat of substitutes
4.5.4.Threat of new entrants
4.5.5.Competitive rivalry intensity
4.6.PESTLE analysis
4.6.1.Political
4.6.2.Economical
4.6.3.Social
4.6.4.Technological
4.6.5.Environmental
4.7.Impact of COVID-19 on left atrial appendage (LAA) closure device market
4.7.1.Pre-covid market scenario
4.7.2.Post-covid market scenario
5. Left Atrial Appendage (LAA) Closure Device Market Analysis, by Product
5.1.Overview
5.1.1.Market size and forecast, by Product
5.2.Endocardial LAA Devices
5.2.1.Definition, key trends, growth factors, and opportunities
5.2.2.Market size analysis, by region,2021-2031
5.2.3.Market share analysis, by country,2021-2031
5.3.Epicardial LAA Devices
5.3.1.Definition, key trends, growth factors, and opportunities
5.3.2.Market size analysis, by region,2021-2031
5.3.3.Market share analysis, by country,2021-2031
5.4.Research Dive Exclusive Insights
5.4.1.Market attractiveness
5.4.2.Competition heatmap
6. Left Atrial Appendage (LAA) Closure Device Market Analysis, by End-use
6.1.Overview
6.1.1. Market size and forecast, by End-use
6.2.Hospitals
6.2.1.Definition, key trends, growth factors, and opportunities
6.2.2.Market size analysis, by region,2021-2031
6.2.3.Market share analysis, by country,2021-2031
6.3.Ambulatory Surgery Centers
6.3.1.Definition, key trends, growth factors, and opportunities
6.3.2.Market size analysis, by region,2021-2031
6.3.3.Market share analysis, by country,2021-2031
6.4.Others
6.4.1.Definition, key trends, growth factors, and opportunities
6.4.2.Market size analysis, by region,2021-2031
6.4.3.Market share analysis, by country,2021-2031
6.5.Research Dive Exclusive Insights
6.5.1.Market attractiveness
6.5.2.Competition heatmap
7. Left Atrial Appendage (LAA) Closure Device Market, by Region
7.1.North America
7.1.1.U.S.
7.1.1.1.Market size analysis, by Product,2021-2031
7.1.1.2.Market size analysis, by End-use,2021-2031
7.1.2.Canada
7.1.2.1.Market size analysis, by Product,2021-2031
7.1.2.2.Market size analysis, by End-use,2021-2031
7.1.3.Mexico
7.1.3.1.Market size analysis, by Product,2021-2031
7.1.3.2.Market size analysis, by End-use,2021-2031
7.1.4.Research Dive Exclusive Insights
7.1.4.1.Market attractiveness
7.1.4.2.Competition heatmap
7.2.Europe
7.2.1.Germany
7.2.1.1.Market size analysis, by Product,2021-2031
7.2.1.2.Market size analysis, by End-use,2021-2031
7.2.2.UK
7.2.2.1.Market size analysis, by Product,2021-2031
7.2.2.2.Market size analysis, by End-use,2021-2031
7.2.3.France
7.2.3.1.Market size analysis, by Product,2021-2031
7.2.3.2.Market size analysis, by End-use,2021-2031
7.2.4.Spain
7.2.4.1.Market size analysis, by Product,2021-2031
7.2.4.2.Market size analysis, by End-use,2021-2031
7.2.5.Italy
7.2.5.1.Market size analysis, by Product,2021-2031
7.2.5.2.Market size analysis, by End-use,2021-2031
7.2.6.Rest of Europe
7.2.6.1.Market size analysis, by Product,2021-2031
7.2.6.2.Market size analysis, by End-use,2021-2031
7.2.7.Research Dive Exclusive Insights
7.2.7.1.Market attractiveness
7.2.7.2.Competition heatmap
7.3.Asia Pacific
7.3.1.China
7.3.1.1.Market size analysis, by Product,2021-2031
7.3.1.2.Market size analysis, by End-use,2021-2031
7.3.2.Japan
7.3.2.1.Market size analysis, by Product,2021-2031
7.3.2.2.Market size analysis, by End-use,2021-2031
7.3.3.India
7.3.3.1.Market size analysis, by Product,2021-2031
7.3.3.2.Market size analysis, by End-use,2021-2031
7.3.4.Australia
7.3.4.1.Market size analysis, by Product,2021-2031
7.3.4.2.Market size analysis, by End-use,2021-2031
7.3.5.South Korea
7.3.5.1.Market size analysis, by Product,2021-2031
7.3.5.2.Market size analysis, by End-use,2021-2031
7.3.6.Rest of Asia Pacific
7.3.6.1.Market size analysis, by Product,2021-2031
7.3.6.2.Market size analysis, by End-use,2021-2031
7.3.7.Research Dive Exclusive Insights
7.3.7.1.Market attractiveness
7.3.7.2.Competition heatmap
7.4.LAMEA
7.4.1.Brazil
7.4.1.1.Market size analysis, by Product,2021-2031
7.4.1.2.Market size analysis, by End-use,2021-2031
7.4.2.Saudi Arabia
7.4.2.1.Market size analysis, by Product,2021-2031
7.4.2.2.Market size analysis, by End-use,2021-2031
7.4.3.UAE
7.4.3.1.Market size analysis, by Product,2021-2031
7.4.3.2.Market size analysis, by End-use,2021-2031
7.4.4.South Africa
7.4.4.1.Market size analysis, by Product,2021-2031
7.4.4.2.Market size analysis, by End-use,2021-2031
7.4.5.Rest of LAMEA
7.4.5.1.Market size analysis, by Product,2021-2031
7.4.5.2.Market size analysis, by End-use,2021-2031
7.4.6.Research Dive Exclusive Insights
7.4.6.1.Market attractiveness
7.4.6.2.Competition heatmap
8.Competitive Landscape
8.1.Top winning strategies, 2021
8.1.1.By strategy
8.1.2.By year
8.2.Strategic overview8.3.Market share analysis, 2021
9.Company Profiles
9.1.Boston Scientific Corporation
9.1.1.Overview
9.1.2.Business segments
9.1.3.Product portfolio
9.1.4.Financial performance
9.1.5.Recent developments
9.1.6.SWOT analysis
9.2.Abbott.
9.2.1.Overview
9.2.2.Business segments
9.2.3.Product portfolio
9.2.4.Financial performance
9.2.5.Recent developments
9.2.6.SWOT analysis
9.3.Lifetech Scientific
9.3.1.Overview
9.3.2.Business segments
9.3.3.Product portfolio
9.3.4.Financial performance
9.3.5.Recent developments
9.3.6.SWOT analysis
9.4.ATRICURE, INC.
9.4.1.Overview
9.4.2.Business segments
9.4.3.Product portfolio
9.4.4.Financial performance
9.4.5.Recent developments
9.4.6.SWOT analysis
9.5.SentreHEART, Inc.
9.5.1.Overview
9.5.2.Business segments
9.5.3.Product portfolio
9.5.4.Financial performance
9.5.5.Recent developments
9.5.6.SWOT analysis
9.6.Medical Device Business Services, Inc.
9.6.1.Overview
9.6.2.Business segments
9.6.3.Product portfolio
9.6.4.Financial performance
9.6.5.Recent developments
9.6.6.SWOT analysis
9.7.Occlutech
9.7.1.Overview
9.7.2.Business segments
9.7.3.Product portfolio
9.7.4.Financial performance
9.7.5.Recent developments
9.7.6.SWOT analysis
9.8.Cardia, Inc.
9.8.1.Overview
9.8.2.Business segments
9.8.3.Product portfolio
9.8.4.Financial performance
9.8.5.Recent developments
9.8.6.SWOT analysis
9.9.Johnson & Johnson Private Limited
9.9.1.Overview
9.9.2.Business segments
9.9.3.Product portfolio
9.9.4.Financial performance
9.9.5.Recent developments
9.9.6.SWOT analysis
9.10.Lepu Medical Technology(Beijing)Co.,Ltd.
9.10.1.Overview
9.10.2.Business segments
9.10.3.Product portfolio
9.10.4.Financial performance
9.10.5.Recent developments
9.10.6.SWOT analysis
10.Appendix
10.1.Parent & peer market analysis
10.2.Premium insights from industry experts
10.3.Related reports
Left atrial appendage (LAA) closure is a medical procedure involving closing or blocking of the opening of the left atrial appendage in order to prevent blood clots from entering the bloodstream. Thus, this procedure helps in preventing strokes in patients with atrial fibrillation, that too without taking blood thinners. The devices which are placed in the heart of patients with atrial fibrillation in order to reduce the chance of thromboembolism from the left atrial appendage are called left atrial appendage (LAA) closure devices.
Forecast Analysis of the Global Market
In recent years, there has been an increase in the incidence of atrial fibrillation due to consumption of a high-calorie diet, smoking, etc., which is expected to be the primary growth driver of the left atrial appendage (LAA) closure device market in the forecast years. Along with this, there has been an increase in the tendency to avoid medications for atrial fibrillations due to their negative side effects and instead opt for installing the left atrial appendage (LAA) closure device. This is expected to push the left atrial appendage (LAA) closure device market further. Also, expanding the healthcare sector, especially in developing countries is predicted to offer numerous investment and growth opportunities to the market in the analysis timeframe. However, the high cost of left atrial appendage (LAA) closure devices may restrain the growth of the left atrial appendage (LAA) closure device market in the forecast period.
Regionally, the left atrial appendage (LAA) closure device market in the North America region is expected to be the most dominant and grow with a CAGR of 20.51% by 2031. The growth and development of advanced solutions in order to treat atrial fibrillation are anticipated to become the primary growth driver of the market in this region. Additionally, the steadily increasing geriatric population in this region is expected to push the market in this region further.
According to the report published by Research Dive, the global left atrial appendage (LAA) closure device market is expected to gather a revenue of $8,218.10 million by 2031 and grow at a 20.97% CAGR in the 2022–2031 timeframe. Some prominent market players include Boston Scientific Corporation, SentreHEART, Inc., Cardia, Inc., Abbott, Medical Device Business Services, Inc., Johnson & Johnson Private Limited, Lifetech Scientific, Occlutech, Lepu Medical Technology Co., Ltd., ATRICURE, INC., and many others.
Covid-19 Impact on the Market
The outbreak of the Covid-19 pandemic has had a massive negative effect on almost all industries and businesses across the world. The left atrial appendage (LAA) closure device market, however, faced a positive impact of the pandemic. The increase in demand for installing these devices to avoid frequent visits to hospitals and clinics led to a massive surge in the growth of this market in the forecast period. Along with this, an increase in the number of cases of atrial fibrillation helped in pushing the left atrial appendage (LAA) closure device market forward, despite the pandemic.
Significant Market Developments
The significant companies operating in the left atrial appendage (LAA) closure device market are adopting numerous growth strategies & business tactics such as partnerships, collaborations, mergers & acquisitions, and launches to maintain a robust position in the overall market, thus helping the market to flourish. For instance:
- In July 2020, WATCHMAN Left Atrial Appendage Closure Device with Delivery System, a LAA closure device developed by Boston Scientific Corporation, a medical device manufacturing company, received FDA approval. With this approval, Boston Scientific Corporation can market its flagship product and further develop this technology so as to cater to the demands of the healthcare sector.
- In August 2020, Conformal Medical Inc., a US-based medical supplier, announced that it had received funding and partnership support from investors such as Fidelity Management and Research Company LLC, an investment and asset management company, and Catalyst Health Ventures (CHV), a financial consultancy firm. This partnership and investment from the two companies will help Conformal Medical Inc., to conduct the all-important trials of its innovative CLAAS device, a state-of-the-art transcatheter left atrial appendage (LAA) closure device.
- In June 2021, Abbott, a multinational medical device manufacturing company, announced that it had received approval from European and Canadian regulators for its new device called Amplatzer Amulet™ Left Atrial Appendage (LAA) Occluder. Amplatzer Amulet™ Left Atrial Appendage (LAA) Occluder is the world’s first steerable delivery sheath which has been developed so as to perform minimally invasive occlusion procedures and this approval will effectively open the European and Canadian markets for this device.
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com